
|
|
|
|
Physics & the Detection of Medical X-Rays
Background: Fluorescence
Without fluorescence Röntgen may have never discovered x-rays. X-rays in his lab struck atoms in a fluorescent material. Then, these atoms emitted visible light . By accident, Röntgen saw this light and began a careful systemic investigation which led to his discovery that these previously unknown rays would show images of bones in his had.
Today, fluorescence is a valuable process in providing much of the artificial light in our buildings, high visibility clothing and many tools for analysis and diagnosis. The least complex explanation of fluorescence is that light with a frequency too high to see enters a material. This light is absorbed and it energy is divided into parts. One part becomes the visible light that we see being emitted by the material; the rest is thermal energy in the material. Thus, high energy light which we cannot see, such as ultraviolet light, is converted into something that we can see.
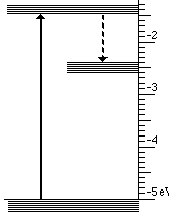
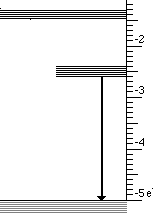
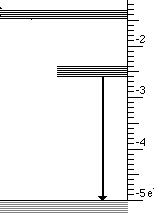
At the atomic level a solid with three energy bands is usually involved. These three bands are the result of impurities which are introduced into a solid. Controlling the type and amount of impurity controls the wavelengths that are absorbed and emitted. This process is illustrated below for the case of material that absorbs ultraviolet light and emits visible light.
This light emission has the following steps
- High energy (UV) photons cause atoms to move from lowest to highest state
- Then an infrared photon is emitted and the atom drops to the intermediate state
- Finally the transition to the lowest state emits visible light
These three steps are illustrated below for visible light.
The illustrations have been created by
Spectroscopy Laboratory Suite interactive
visualization which is part of the Visual Quantum Mechanics
instructional materials.
Perhaps, the most common everyday example of fluorescence involving visible light is the fluorescent tube which provides much of our artificial light. Mercury gas inside the tube emits both visible and ultraviolet photons. The visible photons pass through the coating on the tube and appear as lines in the spectrum of light emitted by the tube, The ultraviolet light is absorbed by the coating on the inner surface of the tube. The material in this coating converts this energy to visible light by the process described above. The color of this light is determined by the material in the coating which determines the energy differences between the various bands.
For x-rays the process is quite similar. The incoming radiation has much higher energy than visible light. An x-ray photon can be converted to one of visible light plus some thermal energy. This process allowed Röntgen to "see" x-rays in his lab. Similarly, Röntgen’s first photographs exposed the film directly by allowing the x-rays to strike the film. The intensity of x-rays needs for this process is very high. Today, image intensifying devices use the very energy of the x-ray to an advantage and provide images with a much lower dose. See the film section.
Thomas S. Warren Museum of Fluorescence
This museum is devoted to fluorescence in mineral and rock. The web site has some nice discussions of the fluorescence process and many nice pictures.
|
|




