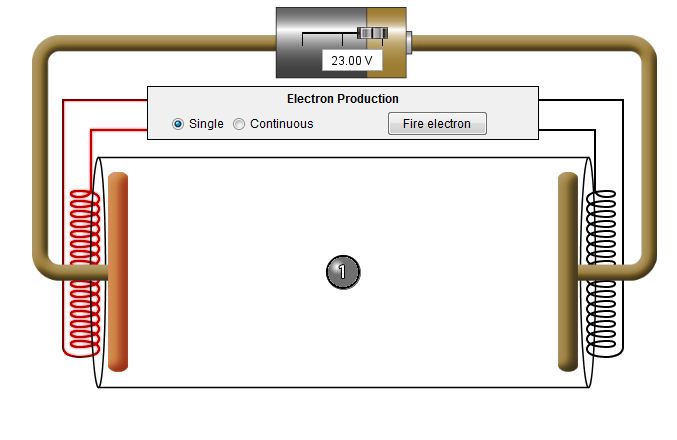
| The curly object at
each end of the tube is a filament made of metal. This
filament on the end of the discharge tube becomes hot. As
the temperature rises and the metal becomes hot, electrons gain
enough energy to become free from the wire that makes up the
filament. |
|
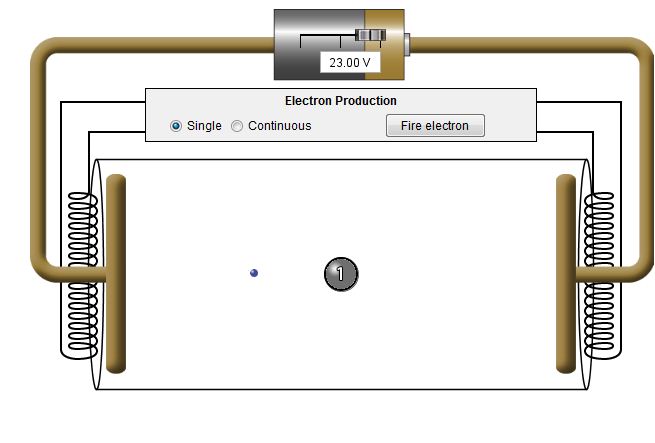
| The two vertical bars next to the
filaments have electrical charge. The electron is repelled
by the one closest to it and attracted by the one on the other
end. So, the electron gains energy. If you change the
voltage on the battery, you will change the amount of attraction
and repulsion and thus change the amount of energy gained by the
electron. The small dot represents this moving electron.(This type
of apparatus was also used in old fashioned TVs and
oscilloscopes. For more about how those devices work click this
link.) |
|
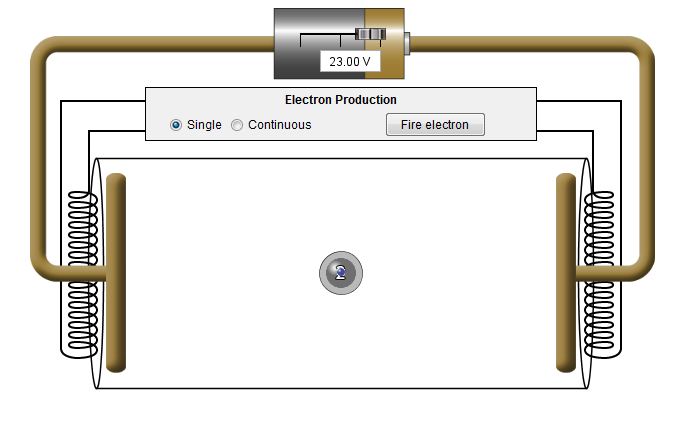
When the electron strikes an atom, it
transfers some of its energy to the atom. For a short time
the atom has an increase in energy. In the visualization
this increase is represented by an extra circle around the atom.
|
 |
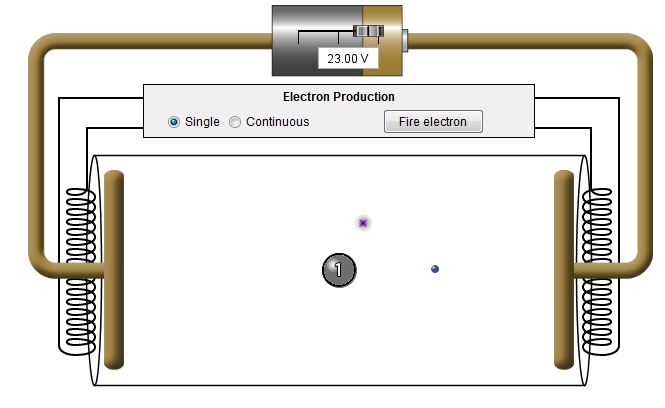
The electron continues its motion but
at a slower speed because it has less energy. The atom gives
up the extra energy that it received from the electron by emitting
a photon of light. In the visualization the photon is
represented by a fuzzy dot. The color of the fuzzy dot
represents the energy of the photon. As described on the
previous screens red indicates low energy visible light while
violet indicates high energy.
|
|



