Understanding the
Spectra Emitted
by Gas Lamps
Goal
- You will use your observations of gas spectra to build a energy level
model of an atom.
Prerequisites
- Conservation of Energy
- Observation and classification of spectra of light sources
- Knowledge of energy level models
Building the Model
- In the last activity, we learned that an electron in an atom loses
energy equal to the difference between two energy values. The energy
lost by the electron appears in the form of light. The energy
difference determines the energy and, thus, the color of light emitted
by the atom. We will now use the Emission Spectra program from
Visual Quantum Mechanics to see how the spectra of light emitted
by gases can help us understand more about the energies in an atom.
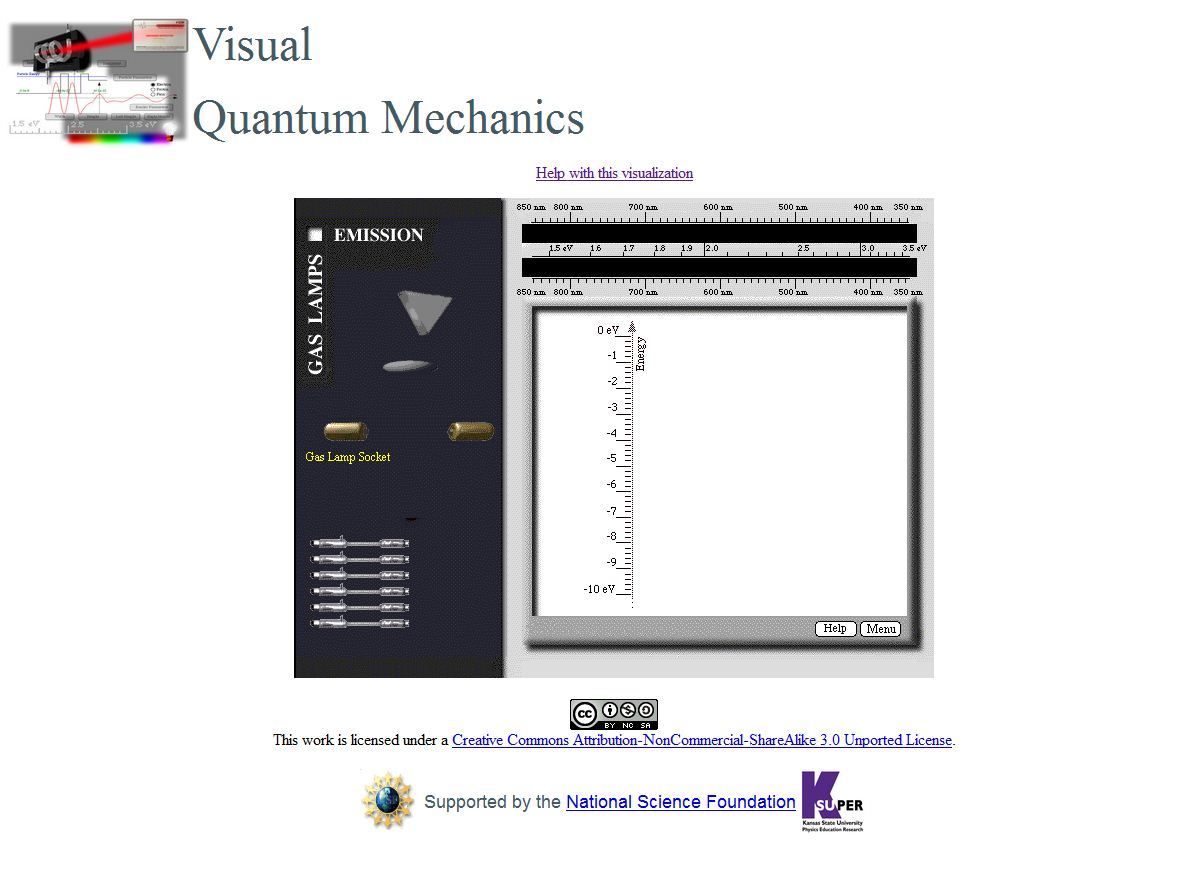
- Use
this link to open the Emission Spectra program. The figure
below shows the screen that should appear.
- In this program, we can �
-
- Select a gas tube and drag it to the socket that is just above the
lamps. Some of the light in the visible portion of the spectrum for
that gas will appear at the top of the screen.
- Add energy levels for an electron in a potential energy diagram
by using the Add Energy Level button.
- Move the energy levels by selecting them at the left of the
vertical energy scale and dragging them to the desired position.
- Move the energy levels by selecting them at the left of the
vertical energy scale and dragging them to the desired position.
- Create transitions (represented by vertical arrow) by selecting
the electronís initial energy on the right of the energy scale. (It
turns green.)
- Drag the transition arrow to the electronís final energy. When you
reach the final energy, it will turn green.
- This process will enable you to create an energy level model of the
light emitting process in an atom. From the results you will be able to
learn
about energy levels in atoms. A colored spectral line on the screen
above the potential energy diagram will indicate the light emitted by
the transition.
If the light is not in the visible region of the spectrum, it will not
appear on the screen.
1 2 3
4 5
6 | Next
page
-