The melting temperature of Copper is 10830C. At temperatures higher than the melting point, Copper vapors are created at high enough concentration so they can serve as an active medium of the laser.
A solid bulk of pure Copper metal is inserted in the middle of the tube before it is filled by the neon gas.
Electric breakdown
is created by high voltage to the electrodes at the ends of the tube. As
a result, the temperature rises inside the tube cavity, until the Copper
evaporates, and the vapor pressure of the Copper is about 0.1 [Torr].
The measured temperature on the outside of the tube can reach 1400-15000C.
During the laser operation only a small fraction of the Copper atoms
are ionized, and they are moving (electrical attraction) toward the ends
of the tube. There, the vapor cool down, and transform to solid metal.
As a result, the amount of Copper vapor in the tube is reduced. After
a few hundred hours of operation, new Copper must be inserted into the
tube.
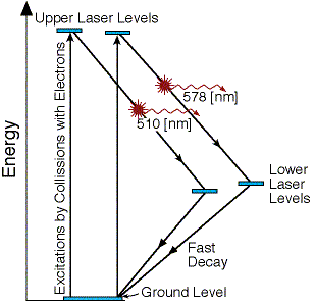
The high voltage pulses applied to the electrodes at the ends of the tube cause the accelerated electrons to collide with the Copper vapor molecules, exciting them into one of the two available high laser energy levels, as seen in figure 6.3.
