Questions
Now, use energy considerations to describe how an atom absorbs a photon from a light source containing all visible energies.
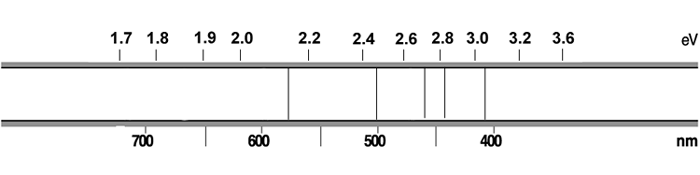
Suppose that we create a beam of light that has photons of energies only in region of 2.0 to 2.4 eV. That beam passes through a tube with hydrogen. Would you see dark lines in the resulting spectrum? Why or why not?
Suppose this light (2.0 - 2.4 eV) passes through nitrogen. Would the resulting spectrum have dark lines? Why or why not? (The nitrogen spectrum is shown below.)