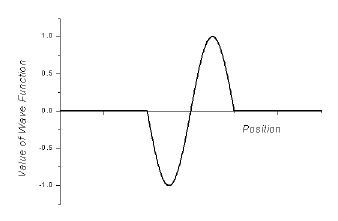
Example of a wave function
for an electron.
To understand how a wave function is related to probabilities
we return (one more time) to the electron interference pattern
and to the plastic waves. The places where electrons are most
likely to be correspond to places where the two waves on plastic
added and the amplitude was large. The locations where the probability
of finding the electrons is zero correspond to locations where
the waves on plastic amplitudes added to zero. In turn the plastic
waves each represent part of an electron’s wave function.
Their addition is the wave function at the screen. A large amplitude
is related to a large probability while a small amplitude goes
with a small probability.