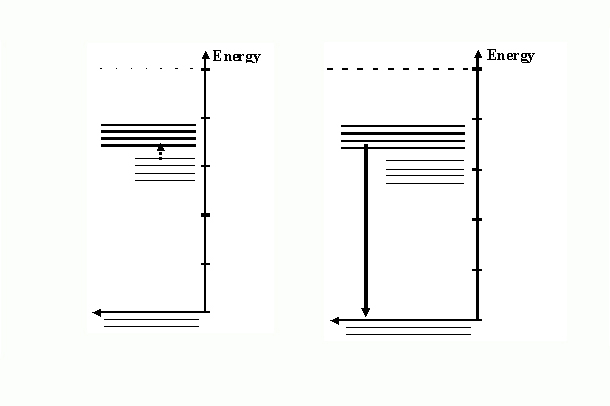
- Describing Phosphorescence
The excited electrons in the glow-in-the-dark toothbrush
do not lose all their energy at once. Instead these electrons
lose enough energy to nearby atoms to make the transition from
the conduction band to the impurity band. In the computer representation
a dashed downward arrow represents this transition. After this
transition occurs, the conduction band turns gray and the impurity
band turns black. This change indicates that electrons have lost
some energy and now have energies associated with the impurity
band. The change in energy is small and is generally in the thermal
energy range.
-
- In fluorescent materials electrons have energies in the impurity
state band for a very short time (10-9 to 10-6 seconds). Then,
they emit light as their energy changes to an energy in the valence
band. As a result, fluorescent materials will only glow while
light of sufficient energy shine on them. In phosphorescent objects,
the electrons remain in the impurity band for awhile. After this
time delay the electrons emit light as their energy changes.
Thus, phosphorescent materials emit light using energy that was
absorbed at an earlier time. When all energy is converted to
light, the object stops glowing in the dark.
-
- The electrons remain with energy in the impurity band because
the physical situation “forbids” a direct transition
from impurity to valence band. To change energy to that of the
valence band the electrons must first gain back the thermal energy
that they lost when they made the transition to the impurity
band. Because the energy is small, it can generally be provided
by the ambient energy in the air. (See the figures below).
-