The Photoelectric
Effect
-
Goals
- Visualize and understand the photoelectric effect
Understand that each element requires a specific photon energy to
release an electron.
Describe why an understanding of this experiment requires the photon
model of light.
Prerequisite
- Energy can transform from one form to another but the total energy is
conserved
Introduction
- The photoelectric effect occurs when light hits a target material
causing some electrons to be released from the target. In 1887 Heinrich
Hertz discovered the photoelectric effect. Surprisingly the results
obtained were unexpected to physicists of that time. In the time of the
experiment, most physicists agreed that light behaved as a wave because
that type of behavior explained the diffraction phenomenon among
other phenomena. But, the wave behavior of light did not explain the
experimental results of the photoelectric effect very well. The
unexpected results were not explained until Albert Einstein used a new
model of light in 1905. But what was unexpected about this
experiment? And what led Albert Einstein to come up with new
explanation? Let’s go back in time to the 1800s and see why this
experiment was shocking.
Optional Reading
- See this
link for more details about the history of this phenomenon.
Begin the Activity
- In this activity you will work with a simulation of the photoelectric
experiment.The simulation is very similar to the real experiment except
you can see the “electrons.” In the experiment you will shine light on a
piece of metal target and analyze the outcomes. To begin follow this link.
- Running it on the Web or downloading it is your choice. Once you
have the simulation ready to go you should have a window that looks like
this figure.
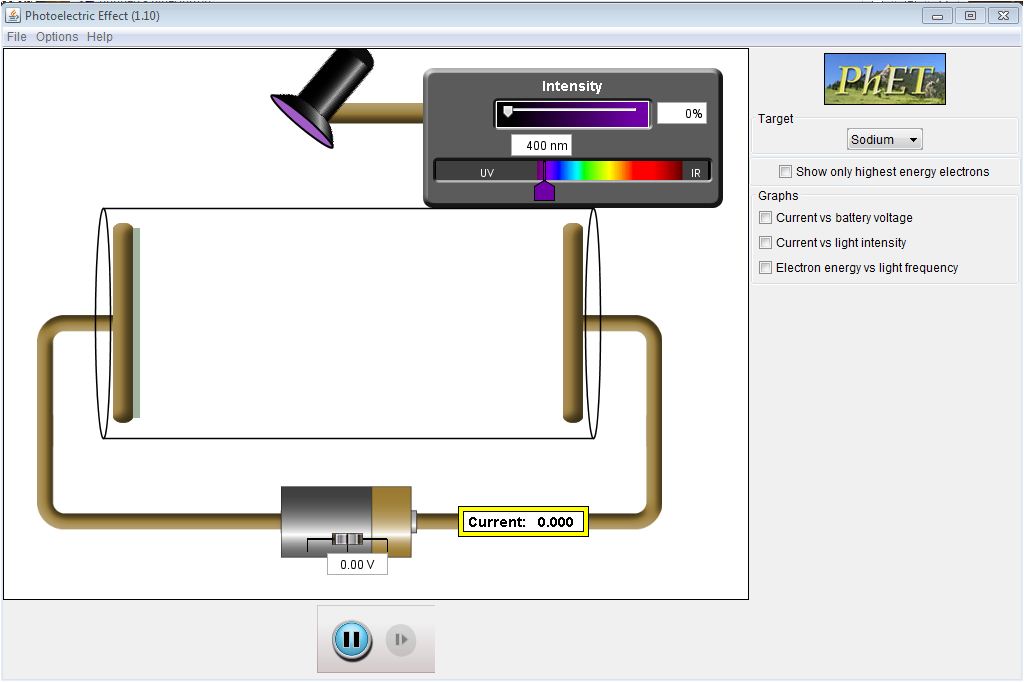
Notes on running the simulation
- As a preliminary step set the voltage on the battery to 0.00 V.
We will leave the voltage at zero throughout this experiment.
- We will not use the graphs that can appear on the right. So,
leave them unchecked.
- For now we wish to look only at the highest energy electrons.
Check that box in the upper right.
- You can change three parameters: light color (wavelength measured in
nanometers), the intensity of the light, and the metal in the target.
- Choose a metal target i.e. Sodium. While keeping the voltage at 0 V,
shine light on it. Change the parameters and record your observations in
the first observations box on the next
page.
1 2 3 4 | Next Page

