Energy Level
Diagrams
Our thoughts on the questions on the previous page.
- How many energy levels are needed to
create one spectral line?
- 2. To emit a photon of light an electron in an atom must change
its energy. Thus it must move from one energy level to another.
- What is the energy of the spectral
line as indicated by the eV scale?
- Your answer will depend on the transition that you set up. So,
if you read the scale correctly, your answer will be correct.
- List the values of the energies that
you created.
- As with the previous question, it depends on your arrange of energies.
- What is the difference in energy
between the atomís initial total energy and its final total energy?
- We cannot determine your answer but we can tell you how to get
it.
- Energy difference = (Initial energy in your transition) - (Final
energy in the transition)
- The final energy is the lower energy in the diagram -- the energy at
the end of the point of the arrow. The inital energy is the other
end of the transition arrow. Be sure to keep trak of all minus
signs.
- How is this energy difference
related to the energy of the light emitted by the atom?
- The energy difference in the atom is equal to the energy of the light
emitted. So, this energy is the same as the energy calculated in
the previous question. That's conservation of energy.
- Move the energy levels up or down
but keep the difference in energy between the electronís final and
initial energy levels constant. Why does the spectral line stay
at the same energy?
- The energy of the photon depends only on the difference
between tow energy levels of the electron. thus, many different
possibilities are available for the energies of the two levels as ling
as the difference between them does not change.
An example
- For this energy level diagram
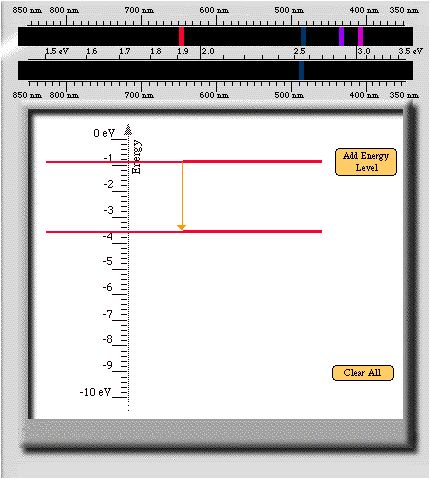
- How many energy levels are
needed to create one spectral line?
- 2
- What is the energy of the
spectral line as indicated by the eV scale?
- About 2.7 eV
- List the values of the energies
that you created.
- -0.9 eV and -3.6 eV
- What is the difference in energy
between the atomís initial total energy and its final total
energy?
- Energy difference = (Initial energy in your transition) - (Final
energy in the transition)
- Energy difference =(-0.9 eV) - (-3.6 eV) = 2.7 eV
- How is this energy difference
related to the energy of the light emitted by the atom?
- The energy difference in the atom is equal to the energy of the
light emitted. So, in this case it is 2.5 eV.
- Move the energy levels up or
down but keep the difference in energy between the electronís
final and initial energy levels constant. Why does the
spectral line stay at the same energy?
- See the answer above for the reasoning. Here are two more
transitions that also create a 2.7 eV photon.
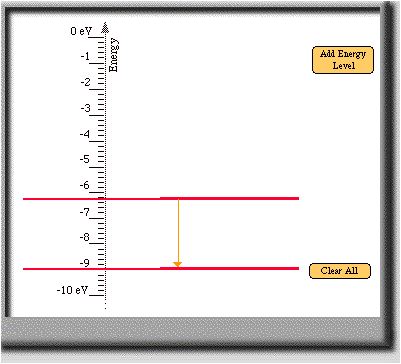
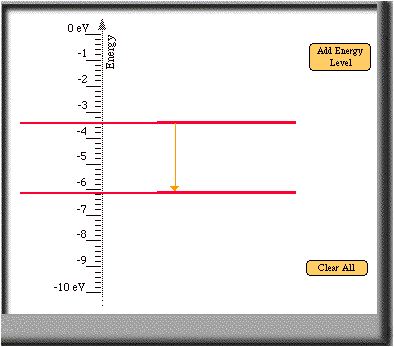
- Previous Page |
1 2 3 4
5 6
| Next page


