Energy Level
Diagrams
More about the Energy Model of the Hydrogen Atom
- Some possible general types of diagrams that students have created
when doing this assignment are shown below.
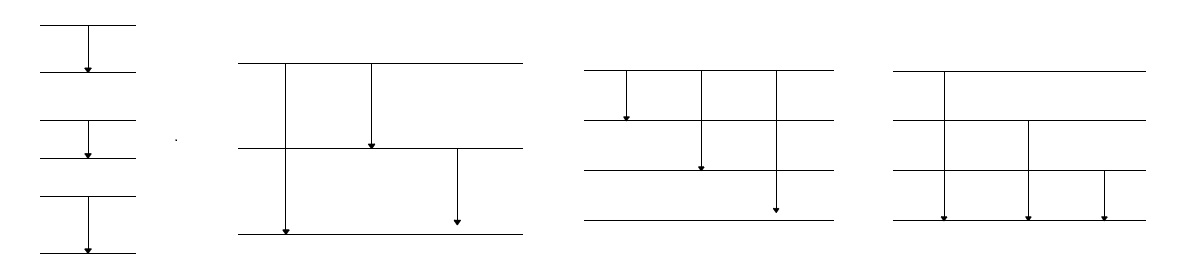
- At this time none of the energy diagrams is more right or wrong than
the others. We do not have enough information to distinguish exactly
what transitions or initial and final energies occur in nature. Our
model is limited by the knowledge that we have. Thus, all sets of
energies and transitions that reproduce the spectrum are equally
correct. (Scientists have more information to help distinguish the
various possibilities, but that is not needed for our purposes.) We can
create energy diagrams that provide all of the spectral lines rather
easily. We need only a few energies to have sufficient transitions for
all of the visible light. From this construction we conclude that an
electron in an atom can have only a few energies. Otherwise we would see
light of many more colors. This conclusion is somewhat surprising. When
an electron moves in an atom, it might seem that the electron could have
any one of many energies. But, nature does not behave that way. Instead
electrons in atoms are limited to a very few discrete energies. We call
these energies the allowable ones.
Energy levels for another gas
- Repeat the steps to determine the energy levels and transitions
necessary to produce the spectral lines emitted by another gas that is
assigned by your instructor.
Sketch the resulting energy level diagram for the second gas think about
how this energy level diagram for the second gas is similar and
different from to the diagram for
hydrogen.
-
Previous Page |
1 2 3
4 5 6
| Next page
