Energy
Level Diagrams
More about the Energy Model of the Hydrogen Atom
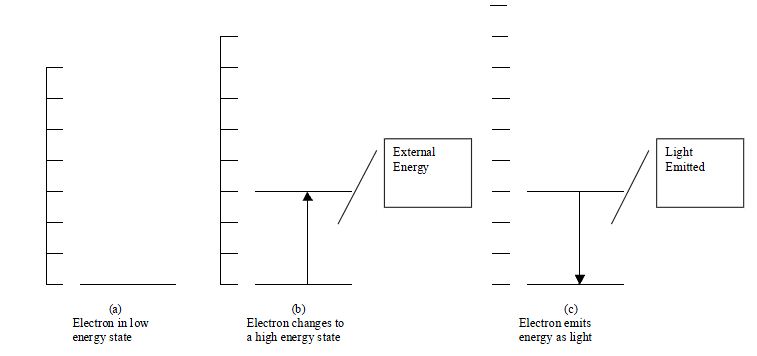
- Up to this point, we have learned that light is produced when
electrons make transitions in atoms. If they have high energy, they
naturally lose it in the form of light as
- they move to a lower energy level. In a normal situation the electrons
will be in a low energy level. They must first be given energy to attain
high energies so that it can
- naturally lose that energy. An external energy source, such as
electricity must supply that energy. This process is illustrated below.
Summary of Energy Levels for gases
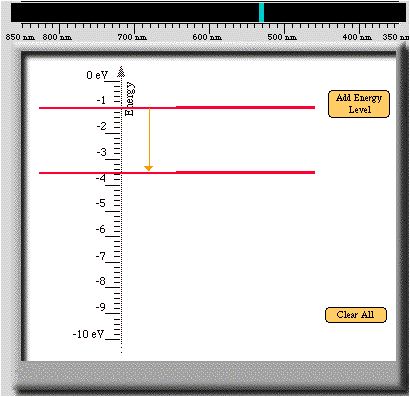
- The larger the external energy provided, the greater the number of
electrons that will obtain higher energies. For example, suppose the
energy difference between two allowed
energies is 2.55 eV. See the figure below.
- In other words, 2.55 eV must be supplied by the external source for a
single electron to change from a low energy to a higher one. Supplying a
larger amount of external energy
does not change the allowed energies. Recall that the allowed energies
for an electron bound to an atom depend on the type of gas atoms found
in the lamp. Supplying a larger
amount of external energy causes a larger number of electrons to possess
the highest allowed energies. Thus, more electrons will make transitions
from higher allowed energies
to lower allowed energies that result in the greater emission of photons
and brighter light.
A different situation occurs if the energy supplied to the atom is not
equal to the difference between energy levels. For the example
illustrated above no transition will occur if
the atom receives less than 2.55 eV. If the atom receives 2.40 eV
of energy, it cannot use the energy.No energy level exists for that
transition to occur. If it gets more than 2.55 eV but less than 5.10 eV
of energy, only one electron can make the transition. When 3.20 eV is
available, the electron may be able to use 2.55 eV and the remaining
0.65 eV will end up as some other form of energy (frequently as motion
of the entire atom).
-
The electrical properties of an atom uniquely determine what energies
its electrons are allowed to have. So, even though the Gas Lamp
Spectroscopy computer program allows
you to adjust the energies available to the electrons, these energies
are fixed at very specific values by the electrical properties of the
atom. Because these electrical properties are different for
each of the chemical elements, the atoms of each of the elements have a
unique set of energies. Thus, the light given by any hydrogen atom is
the same as every other hydrogen atom. However, that light is
different from the ligth emitted by every helium atom. Thus the light
emitted by a material can be used to determine the type of elements
present. This property is used to learn about the composition of distant
stars as well as substances on earth
Previous Page |
1 2 3
4 5 6