Changing Energies --- Transitions
Conservation of energy tell us that:
Electron energy before = Electron energy after + Light (photon) energy
Each time an electron decreases its energy it emits one photon. Thus, by looking at the energy of photons we can learn about what is happening in an atom. From the light that they see, the students reach conclusions about the atom. This process allows them to build models of the atom.
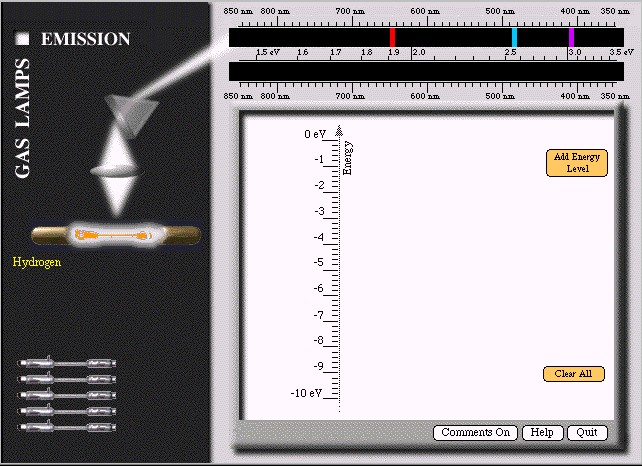
We will now use Spectroscopy Lab Suite to see how the spectra of light emitted by gases can help us understand more about the energies in an atom. Select Emission under Gas Lamps. Figure 1.2 shows the screen that appears. In this program, we can

Create transitions (represented by a vertical arrow) by selecting the electron's initial energy on the right of the energy scale. (It turns green.) Drag the transition arrow to the electron's final energy. When you reach the final energy, it will turn green. You may move any of the energy levels after you have created a transition. This process will enable you to create an energy level model of the light emitting process in an atom. From the results you will be able to learn about energy levels in atoms. A colored spectral line on the top black screen will indicate the light emitted by the transition. If the light is not in the visible region of the spectrum, it will not appear on the screen.
We can create energy diagrams that provide all of the spectral lines rather easily. We need only a few energies to have sufficient transitions for all of the visible light. From this construction we help students realize that an electron in an atom can have only a few energies. Otherwise we would see light of many more colors. This conclusion is somewhat surprising. When an electron is bound to an atom, it might seem that the electron could have any one of many energies. But, nature does not behave that way. Instead electrons in atoms are limited to a very few discrete energies.