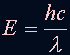
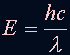
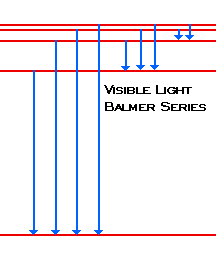
| When the electron changes from n=3 or above to n=2, the photons emitted fall in the Visible Light region of the spectra. We call these lines Balmer's Series. |
 |
The relation of the energy levels and wavelength is: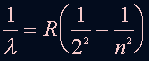 where n=3,4,5 ... and R = 1.097 x 10^7 (1/m) |
| When the electron changes from n=2 or above to n=1, the photons emitted fall in the Ultra Violet region of the spectra. We call these lines Lyman's Series |
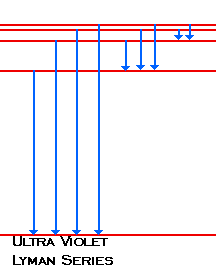 |
The relation of the energy levels and wavelength is: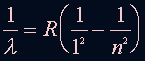 where n=2,3,4 ... and R = 1.097 x 10^7 (1/m) |
|
|
ENDS EMISSION
STARTS IONIZATION |
|