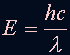NOTE:
Throughout this tutorial, there will be a series of boxes and choices for you to
fill out. This will be an aid, similar to a worksheet, to allow you to
think out your answers more clearly. We are also interested in your opinions
and answers. All forms will be sent anonymously (we don't request your e-mail or name),
so feel free to write however much you feel is necessary to help you. At
the current time, your information can be accessed to you again by pressing the
back button on your browser.
|
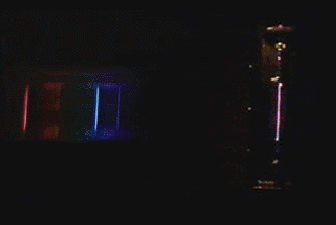 For non Java Browsers: (Close up image)
For non Java Browsers: (Close up image)
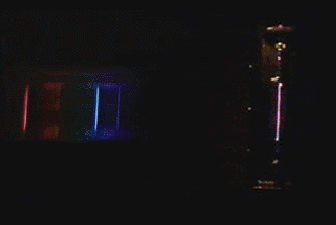 For non Java Browsers: (Close up image)
For non Java Browsers: (Close up image)